CSIR-NAL develops Non-Invasive Ventilator to treat COVID-19 patients
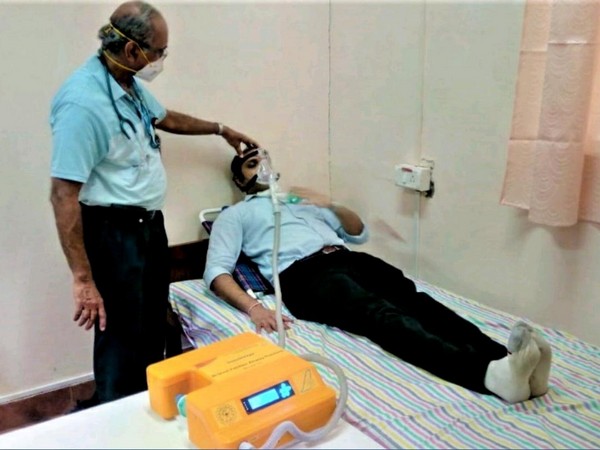
New Delhi [India], May 12 (ANI): In a record time of just 36 days a non-invasive BiPAP Ventilator ‘SwasthVayu’ has been developed by CSIR – National Aerospace Laboratories (NAL), Bangalore, a constituent of lab of CSIR to treat COVID-19 patients.
Dr Shekhar C Mande, DG CSIR said that CSIR NAL team has enabled a spin-off technology based on its expertise in the aerospace design domain. The system has undergone stringent biomedical tests and beta clinical trials at NAL Health Centre.
Jitendra J Jadhav, Director CSIR -NAL said the non-invasive ventilator will be ideal to treat moderate or mid-stage severe COVID-19 patients who do not require intubation and invasive ventilation. “Based on global experience and specific inputs from pulmonologists in India and abroad, CSIR -NAL developed BIPAP non-invasive ventilator with externally connected oxygen concentrator which will be ideal to treat moderate or mid-stage severe COVID-19 patients who do not require intubation and invasive ventilation,” he said.
The system has been certified for safety and performance by National Accreditation Board for Testing and Calibration Laboratories (NABL ) accredited agencies and undergone stringent biomedical tests. Officials said that the major advantage of this machine is that it does not require any specialised nursing for usage.
They said it is cost effective, compact and configured with majority of indigenous components and is ideal for treating COVID -19 patients in wards, makeshift hospitals, dispensaries and homes in current Indian COVID-19 scenario.
It’s unique features like spontaneous, CPAP, timed, auto BIPAP modes with provision to connect Oxygen concentrator or enrichment unit externally alleviate the fear of the virus spread. BiPAP non-invasive ventilator is a microcontroller-based precise closed-loop adaptive control system with a built-in biocompatible “3D printed manifold and coupler” with HEPA filter (Highly Efficient Particulate Air Filter).
CSIR -NAL is in process of taking it forward with the regulatory authorities for the approval and expected to get permission shortly. It has already initiated dialogue with major public/private industries to partner for mass production. (ANI)
















