By Shalini Bhardwaj
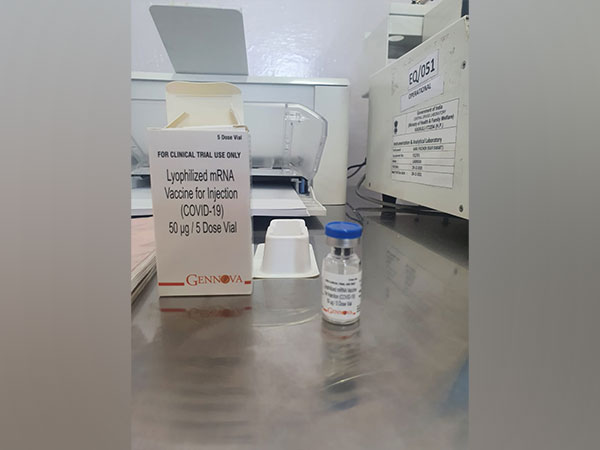
New Delhi [India], March 18 (ANI): Pune-based Gennova Biopharmaceuticals has submitted the phase-II and phase-III trials data of India’s first mRNA COVID vaccine to the regulator Drugs Controller General of India (DCGI) on Friday, said sources. The company has also developed an Omicron-specific vaccine which will be tested on humans for efficacy and immunogenicity.
Earlier the DG, Indian Council of Medical Research (ICMR) Dr Balram Bhargava informed that this vaccine is going to be useful in the future for the treatment of other diseases as well and India is heading towards becoming a vaccine superpower. “India is heading towards becoming a vaccine superpower and the fact that these vaccines are going to be available for other diseases,” said Dr Bhargava.
Dr V K Paul, Member (Health) Niti Aayog on the importance of mRNA vaccine had said, “This mRNA platform of vaccine is an asset today in wake of COVID and also beyond it for other diseases. It could be malaria, dengue or TB. There are so many diseases for which we are still hunting for affordable and effective vaccines.” Gennova Biopharmaceuticals has submitted phase-II and phase-III data of mRNA vaccine. The recommendations about vaccines will come only after evaluation by the Subject Expert Committee (SEC).
Meanwhile, India started vaccinating children aged 12 to 14 years as it expands its COVID-19 vaccination coverage on March 16. The children in the said age group are administered Corbevax vaccine manufactured by Hyderabad-based Biological E. It is India’s first indigenously developed Receptor Binding Domain (RBD) protein sub-unit vaccine against COVID-19.
Additionally, all above 60 years of age are now eligible for Precaution Dose, as the condition of comorbidity for this age group has been removed. The Precaution Dose (same as the previous two doses) is to be administered after 9 months (36 weeks) after the date of the second vaccination. (ANI)